Science puts a spotlight on leafhopper species
[ad_1]
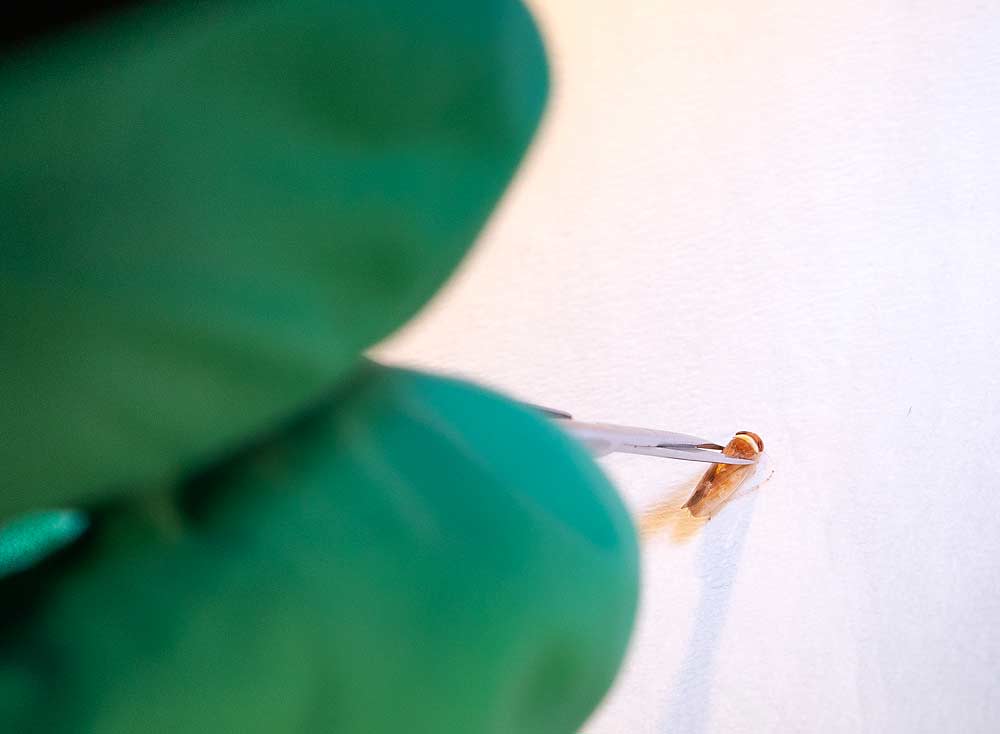
The X disease epidemic in Northwest stone fruit orchards catapulted a once anonymous leafhopper, Colladonus reductus, into the research spotlight.
While several leafhopper species can carry the devastating X disease phytoplasma, C. reductus accounts for most vector activity in Washington today.
“It seems that C. reductus is very, very good at transmitting strain 3,” said Washington State University pathologist Scott Harper, referring to one of the two strains found in the Northwest today. Strains 2 and 3 differ from the strain that drove previous outbreaks in the Western growing region and from the strain found in the Eastern U.S. Strain 3, in particular, appears to spread more aggressively due to the pathogen/vector compatibility.
That’s one of many findings emerging as scientists dive deep into understanding the pathogen/vector complex to develop strategies that move X disease control from emergency response to sustainable management.
“The goal is to develop an IPM approach,” said WSU entomologist Tobin Northfield. Instead of spraying the same products prophylactically and risking resistance, researchers are developing phenology models to optimize spray timing and weed management strategies to make orchards a less appealing habitat for the hoppers. “Spraying when we need to and creating a more inhospitable environment can be a more efficient approach.”
Currently, over a dozen research projects are underway, funded with $1.6 million in federal grants and $540,000 in grants from the Washington and Oregon cherry industries.

Transmission
Scientists already knew that the phytoplasma a leafhopper slurped up while feeding on an infected tree takes time to move from the insect’s guts to its mouthparts — where it can be transmitted to other plants on future feedings. During Washington’s last outbreak in the 1960s, entomologists estimated it took about a month.
“But they only had visual symptoms of the plant getting infected, they didn’t have the DNA tools we do now,” said Adrian Marshall, a postdoctoral researcher with the U.S. Department of Agriculture’s Wapato, Washington, lab.
Therefore, researchers based management around the idea that wiping out the leafhoppers every three weeks would be sufficient, he said.
That’s due for some fine-tuning.
Last summer, Marshall launched a study to measure today’s transmission by putting lab-reared adult leafhoppers in a bag on a branch of a severely infected cherry tree for two or three days. After that forced feeding, he brought the insects back to the lab and decapitated them to test separately for phytoplasma in the head and the body six, 10 and 17 days later.
“After six days, their bodies would test positive but their heads did not, so they could not transmit,” he said. After 10 days, a few heads tested positive, but by day 17, “50 percent were positive in the head and body.”
Marshall called this a worst-case scenario — given the extremely high level of phytoplasma in the host tree — in terms of the percent of leafhoppers capable of transmitting the disease. In typical orchard sampling, only a small percentage of C. reductus leafhoppers caught are vectors.


“So, please remove your super-infected trees,” he said. “Spraying keeps your leafhoppers low, but if you don’t have those super-infected trees, you’ll have way less of a problem.”
He plans to continue the trial looking at trees with lower infection levels more typically found in commercial orchards and is working with Harper, the WSU pathologist, to see if different strains of the X disease phytoplasma have different transmission timelines.
Pairing this leafhopper-as-vector timeline with phenology models in development should allow growers to dial in spray programs, Marshall said.
Habitat hacking
Researchers also recently demonstrated that removing root suckers may reduce the risk of X disease transmission. Leafhoppers live in the groundcover, and low-lying suckers act like a ladder for them to move into the tree canopy. In a small trial, removing suckers reduced the number of leafhoppers in the canopy by half, Marshall said.
When trees are already infected, suckers can contain very high levels of the pathogen as well, Harper said, increasing the odds that leafhoppers feeding on them would pick it up.
To make the groundcover itself less attractive to leafhoppers, Northfield recommends removing broadleaf weeds by using herbicides or planting competitive grass cover.
“Our assumption is they are picking it up as nymphs and transmitting it as adults,” he said. “The fastest approach is to starve nymphs by removing their host plants.”
Analysis of C. reductus gut contents shows that infected leafhoppers spend a lot of time feeding on dandelion and mallow, said USDA entomologist Rodney Cooper, director of the Wapato lab.
“It reinforces the message that if you control those weeds, you reduce the risk of X disease in orchards,” he said.

In lab colonies, C. reductus can complete its life cycle on dandelion, Cooper said. Grass, on the other hand, is a poor host for both leafhoppers and phytoplasma. In one commercial orchard that planted grass, Northfield’s team has seen the leafhopper population reduced by half, he said.
“We’re going to track that over time” to confirm the findings, he said. “It’s not perfect, but a lot of growers are using sprinklers anyway and watering weeds in the drive row, so I don’t see any way it’s not economically beneficial.”
Broadleaf herbicide treatments also drive a similar reduction in leafhopper numbers, according to results in the first year of trials.
Looking for X disease in weeds themselves can indicate whether there are high levels of transmission in the orchard environment, Harper said, but the weeds don’t host as much phytoplasma as do infected trees.
When he looks for the pathogen in the natural landscape around orchards, he does not find the modern strains. “It’s spilling out of the orchard into the wild, not coming the other way,” he said.

Clues in the DNA
Meanwhile, scientists continue their search for potential biocontrols. César Reyes Corral, a WSU graduate student working with Northfield, has found two species in the Pacific Northwest that parasitize leafhoppers: the big-headed fly and the pincer wasp.
“We know they are here, but we need to figure out how much of an impact they represent in the leafhopper populations,” Reyes Corral said. To that end, he’s developing a genetic screening tool that can test leafhopper tissue for the DNA of multiple parasitoids in one test.
“We’re talking about spraying every two weeks for months, so the idea of a biocontrol agent is very exciting, but this is in the very early stages,” he said.
USDA geneticist William Walker and his colleagues have recently sequenced the genome of C. reductus and C. geminatus, another, less common leafhopper vector. They also mapped the transcriptome — a look at all the genes actively being translated into proteins — in infected versus uninfected leafhoppers of both species and in the heads versus the bodies.
“We’re looking to see how the X disease pathogen exploits the leafhopper to facilitate its own life cycle,” Walker said. “That could give us targets for manipulation such as biopesticide targeting or other (forms of) disruption to halt the life cycle in the insect.”
That’s not as far-fetched as it sounds. A sprayable biopesticide that uses a gene-silencing technique known as RNA interference to inhibit a key enzyme in the Colorado potato beetle was recently registered for use. If researchers can identify key genes that could shut down a pathway that turns the leafhopper into such a competent vector, a similar approach could be viable, Walker said.
Every approach counts. Fighting the disease is a numbers game, Northfield said.
“We are just chipping away at leafhopper density with every tool we have,” he said.
That includes — crucially — removal of infected trees.
“We want to reduce the proportion of them that are damaging,” Northfield said. “If we removed the phytoplasmas from our system, we wouldn’t care about leafhoppers anymore.”
—by Kate Prengaman
Lots of leafhoppers
There are lots of leafhopper species in Northwest orchards, and only a few can become villainous vectors of X disease.
“Many, like those little green ones, are not a problem,” said Washington State University Extension specialist Tianna DuPont during the North Central Washington Stone Fruit Day in January.

For help distinguishing C. reductus from its cousins and distant relatives, WSU developed an app with photos and descriptions of common leafhoppers you may see on your sticky traps. It also has tools to help with scouting for X disease symptoms.
Search “Little Cherry Scouting Guide” in your app store.
—K. Prengaman
[ad_2]

















