In a recent study published in the journal Antioxidants, researchers investigated the mechanisms by which Resveratrol, a natural phenolic compound, can prevent and attenuate Alzheimer’s Disease (AD). They used the BV2 microglial cell lines established from C57BL/g transgenic murine models to elucidate the mechanistic benefits of Resveratrol against glia activation by proinflammatory monomeric C-reactive protein (mCRP). Their results highlight that Resveratrol inhibits lipopolysaccharides (LPS) and mCRP-induced-cyclooxygenase-2, thereby preventing the release of proinflammatory cytokines. It further upregulates the expression of the antioxidant enzymes, namely Cat and Sod2. Together, these results provide the mechanistic underpinning for the benefits of Resveratrol in combatting and controlling AD.
 Study: Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Image Credit: Aimee Lee Studios / Shutterstock
Study: Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Image Credit: Aimee Lee Studios / Shutterstock
What is Resveratrol?
Resveratrol, commonly found in red grapes and their derivatives (e.g., red wine), is a natural phenolic compound belonging to the stilbene family. Extensive preclinical research into the metabolite has revealed its potent anti-inflammatory, anti-neurodegenerative, antioxidant, and anti-aging properties. It is a common compound synthesized by more than 70 known plant species as a stress-response mechanism.
Recent Resveratrol studies in animal models have shown that trans-resveratrol is capable of crossing the blood-brain barrier, suggesting that it might perform neuroprotective functions. This is augmented by the fact that older men and women who drink moderately are consistently observed to have lower dementia risk compared to lifetime abstainers. Unfortunately, the human body does not produce Resveratrol, and therapeutic dosages (150-250 mg/d) can only be acquired through oral supplementation.
Scientists have attempted to elucidate this compound’s impacts on neurodegenerative and non-neural medical conditions to arrive at its mechanistic underpinning. However, Resveratrol’s mechanisms of action in humans remain a mystery, given the inconclusive findings of said studies. The compound is both hormetic and hydrophobic, limiting its absorption and bioavailability. Researchers have circumvented this by developing novel nanocarrier-based delivery systems showing significant promise in cancer- and neurotherapy. Murine models have further suggested that Resveratrol could substantially reduce oxidative stress and improve neurodegenerative outcomes via tumor necrosis factor α (TNFα) downregulation. However, these claims remain to be tested.
Understanding the mechanisms by which Resveratrol exercises its neurological benefits might allow for the development of new interventions aimed at preventing or managing Alzheimer’s Disease (AD). It would further inform future clinical trials of the safe dosage range, given that the chemical can be cytotoxic in high concentrations.
About the study
In the present study, researchers attempt to evaluate the antioxidant protection mechanism of Resveratrol using BV2 microglia, which have been activated by monomeric C-reactive protein (mCRP). mCRP activation and overexpression are vital traits of most inflammation-activated diseases, and its prevention may delay or even reverse conditions like AD that progress in part due to inflammatory stress.
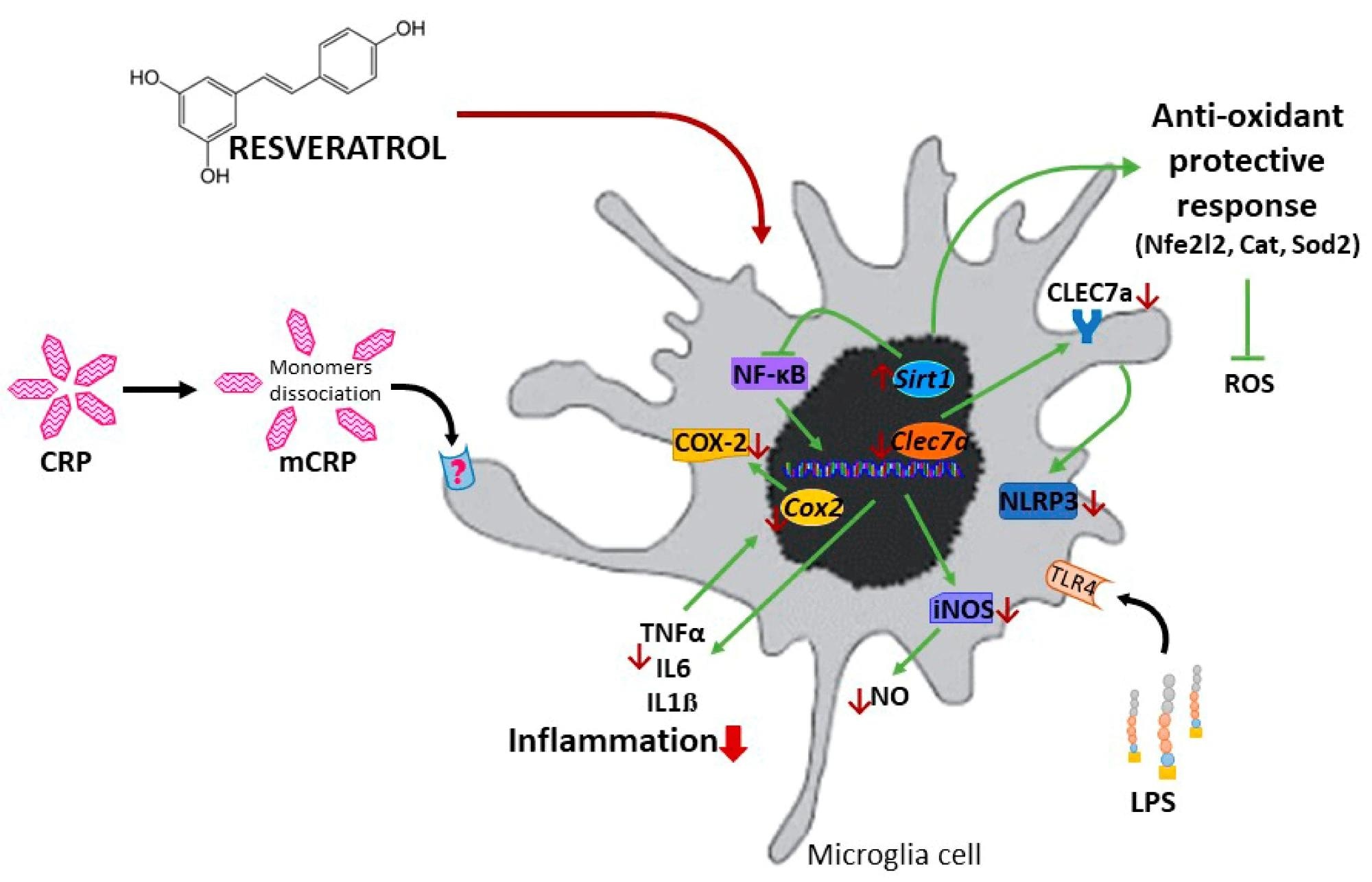 Schematic representation of the protective mechanisms of resveratrol against the proinflammatory agent mCRP and LPS.
Schematic representation of the protective mechanisms of resveratrol against the proinflammatory agent mCRP and LPS.
The BV2 cell line used herein was established from C57BL/6 transgenic mice microglia, an established model for studying brain inflammation. mCRP was generated from pure CRP protein via urea/ ethylenediaminetetraacetic acid (EDTA) chelation, followed by dialysis. Escherichia coli 026:B26 was used as a lipopolysaccharide (LPS) strain. Resveratrol treatments on these primary cell cultures varied between 10-50 µM. mCRP assays utilized mCRP at 50 µg/mL. To avoid astrocyte damage, primary glial cultures were not subjected to nutrient (serum) starvation.
Nitric oxide generation by glial cultures was determined using the colorimetric Griess reaction. The Enzyme-Linked Immunosorbent Assay (ELISA) was used to detect and measure tumor necrosis factor-alpha (TNF-α) and interleukin one-beta (IL1 ß) expressed in ng/mL and pg/mL, respectively. Western blotting assays were used to detect and identify other protein products produced by BV2 cells. BV2 cell RNA was then extracted and subjected to Real-Time Quantitative Polymerase Chain Reaction (qPCR) to determine relative gene expression.
Finally, the immunofluorescence assay was used to measure the impact of Resveratrol on BV2 cell expression. Statistical analyses comprised two-way analysis of variance (ANOVA) and the Shapiro–Wilk test.
Study findings
Resveratrol was observed to significantly inhibit and reduce TNF-α production induced by mCRP and LPS, elucidating and validating its anti-inflammatory properties. The compound was further observed to suppress the activation of the nitric oxide pathway, preventing the generation of reactive oxygen species (ROS).
Activation of the NLR family pyrin domain containing 3 (NLRP3) gene was also observed to be inhibited by Resveratrol. NLRP3 is the gene responsible for producing the cryopyrin protein, a crucial microglia cell sensor inflammasome activated during oxidative stress. Nuclear factor-κB (NF-κB) and Nos2 were observed to be downregulated on the addition of Resveratrol. Finally, Resveratrol was found to induce the expression of antioxidant genes, including Sirt1 and Nfe2I2.
In summary, Resveratrol’s anti-AD effect was shown to arise due to a combination of oxidation suppression and antioxidant expression.
Conclusions
In the present study, researchers investigated the mechanisms by which Resveratrol, a plant metabolite found in over 70 species, can promote positive neurodegenerative outcomes, especially in AD. They used a combination of in vitro cell cultures, ELISAs, western blotting, and qPCR and revealed that Resveratrol both suppresses the generation of ROS and enhances the expression of antioxidant-protecting genes.
“Resveratrol protected against the polarization of BV2 microglia into an activated phenotype induced by two critical proinflammatory agents, LPS and mCRP. The characterization of mCRP proinflammatory and pro-oxidant mechanisms in BV2 microglia showed the activation of the inflammatory/oxidative cascades of nitric oxide, NLRP3 inflammasome and COX-2 in this novel in vitro model. Resveratrol protective mechanisms against mCRP required the modulation of SIRT1, Nrf2, and NF-ĸB pathways that reduced downstream inflammatory mediators and, most notably, induced antioxidant enzymes. Resveratrol protective mechanisms against activation to proinflammatory phenotype by mCRP was confirmed in primary mixed glial cultures.”
These findings highlight the potential for Resveratrol in future AD preclinical testing. Resveratrol and similar plant-derived metabolites may allow for the development of future clinical interventions against currently incurable diseases such as AD. However, extensive clinical trials are required to assess the effectiveness of Resveratrol on other oxidation-inducing genes and to arrive at a dosage that is safe for human use.
Journal reference:
- Bartra, C., Yuan, Y., Vuraić, K., Slevin, M., Pastorello, Y., Suñol, C., & Sanfeliu, C. (2024). Resveratrol Activates Antioxidant Protective Mechanisms in Cellular Models of Alzheimer’s Disease Inflammation. Antioxidants, 13(2), 177, DOI – 10.3390/antiox13020177, https://www.mdpi.com/2076-3921/13/2/177